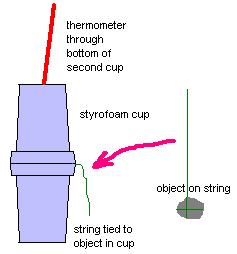
Obtain a thermometer, two styrofoam cups, and an object. Some of the objects which are using might include
nut coral silver cylinder fishing mass basalt pair of brown cylinders golden cube grey cube dented cube w/ square handle
| Description of object: | _________________ | |
| The mass of the styrofoam cup | mc | __________ |
| The mass of the styrofoam cup with water | mc+w | __________ |
| The mass of the water mc+w - mc | mw | __________ |
| The mass of the object | mx | __________ |
| The start temperature of the object | tx | __________ |
| The start temperature of the water | ti | __________ |
| The highest final temperature of the water and object | tf | __________ |

Note that the specific heat capacity of water, cw, is 1 calorie/(gram ·°C)
First law of thermodynamics: Energy is conserved. Heat is conserved. The object cooled down. The water warmed up. The object lost heat. The water gained heat. The heat lost by the object to the water is equal to the heat gained by the water. Heat is measured in calories. The letter Q is used for heat in calories.
Qlost by object = Qgained by water
mxcx[tx - tf] = mwcw[tf-ti]
( mx )cx[tx -( tf )] =( mw )(cw)[( tf )-( ti )] ( )cx[tx -( )] =( )(1)[( )-( )]
Solve for cx, the specific heat capacity of the object.
cx = ___________
After performing the experiment on one material, obtain a second material by trading objects with another group. Make the same basic measurements, calculations, and report the result.
| Description of second object: | _________________ | |
| The mass of the styrofoam cup | mc | __________ |
| The mass of the styrofoam cup with water | mc+w | __________ |
| The mass of the water mc+w - mc | mw | __________ |
| The mass of the object | mx | __________ |
| The start temperature of the object | tx | __________ |
| The start temperature of the water | ti | __________ |
| The highest final temperature of the water and object | tf | __________ |
( mx )cx[tx -( tf )] =( mw )(cw)[( tf )-( ti )] ( )cx[tx -( )] =( )(1)[( )-( )]
cx = ___________
Use the following table, if appropriate, to make an educated guess as to the materials you were working with if you were working with a metal. You can use density to guide you when in doubt. The density is the mass divided by the volume. The volume of a cube is length×width×height, the volume of a cylinder is p×(radius)2×Length
| material | s.h.c. in cal/(g ·°C) | Density g/cm3 |
|---|---|---|
| aluminum | 0.215 | 2.7 |
| basalt | 0.24* | |
| brass | 0.092 | 8.4 |
| copper | 0.092 | 8.96 |
| glass, crown | 0.161 | 2.5 |
| iron | 0.107 | 7.87 |
| lead | 0.031 | 11.3 |
| magnesium | 0.245 | 1.74 |
| steel | 0.107 | 7.8 |
| zinc | 0.093 | 7.14 |
* Based on experimental data taken in physical science laboratory.
If you can determine the material, then work out the percentage error for your experiment.
Do the homework on page 45 of the lab book.
SC 130 Home page
Lee Ling's Courses Home Page
![]() To COM-FSM Home Page
To COM-FSM Home Page